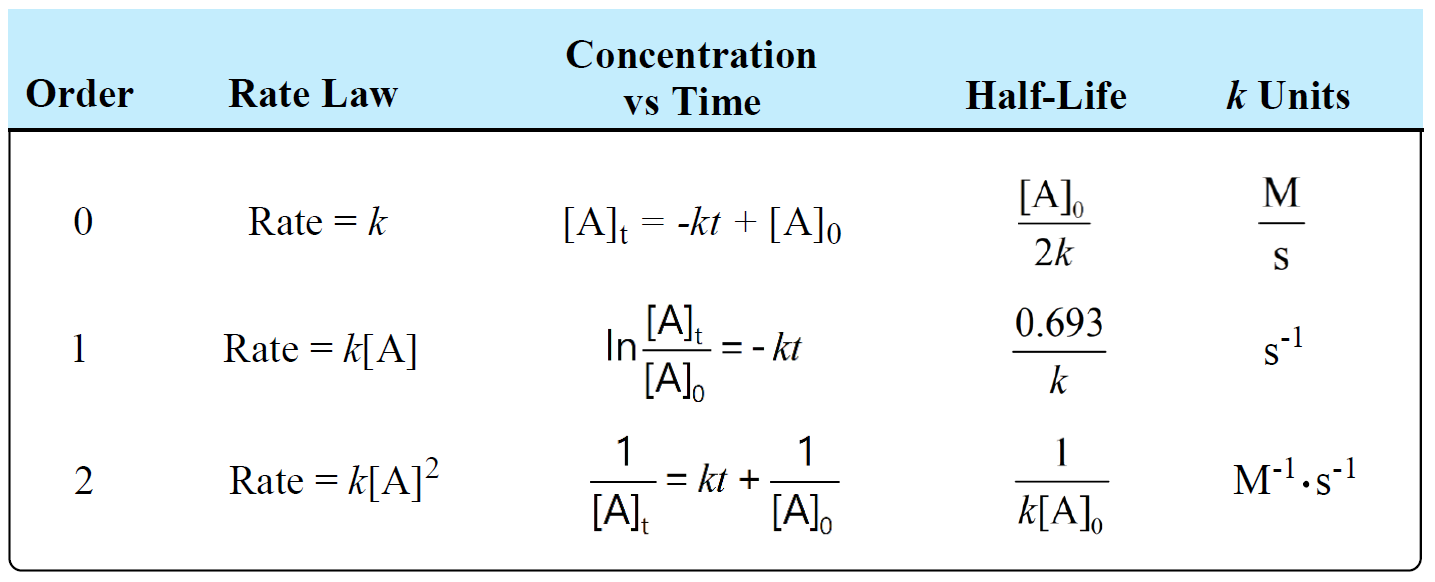
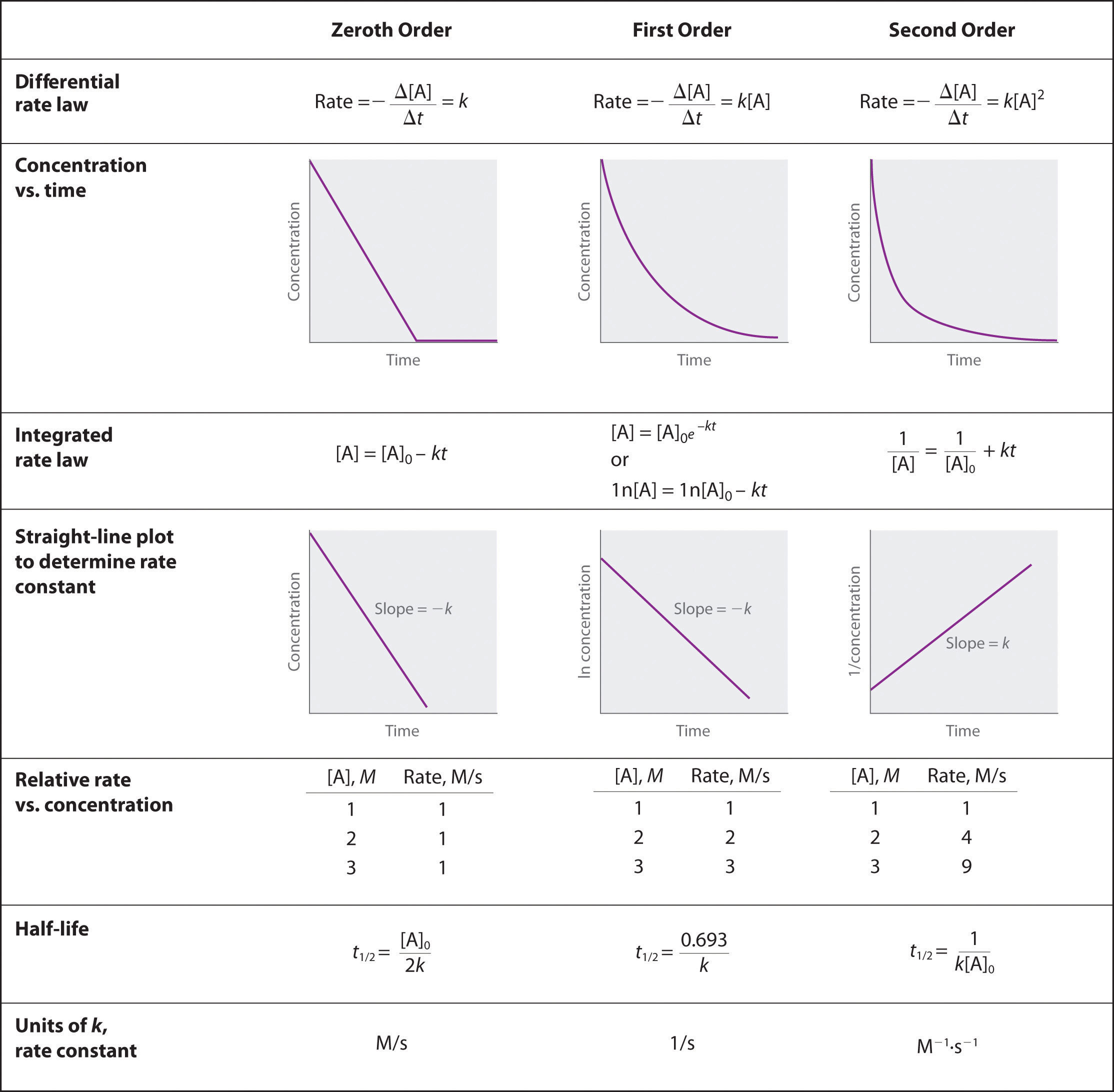
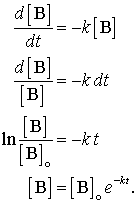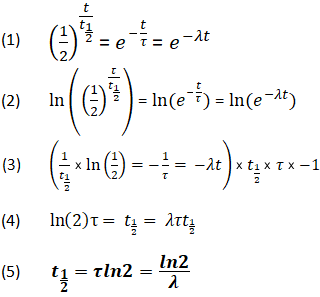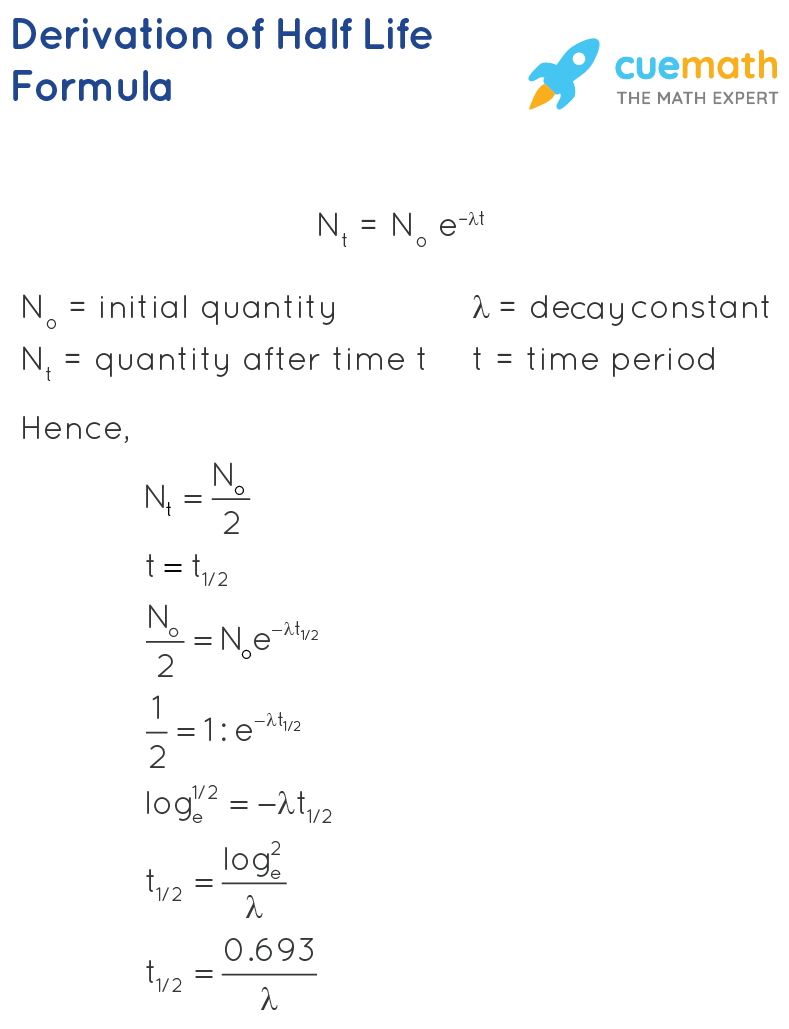half life formula for zero order reaction
A reactions half-life formula changes depending on the order of the reactions. Another method for determining the order of a reaction is to.
Principles And Kinetics Of Drug Stability Phr 416 Ppt Video Online Download
In some cases we need to know the initial.

. Half-life of radioactive substance is 6 h. Half life formula for nth order reaction. For the 1 st.
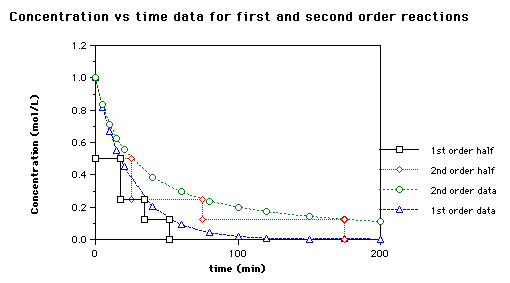
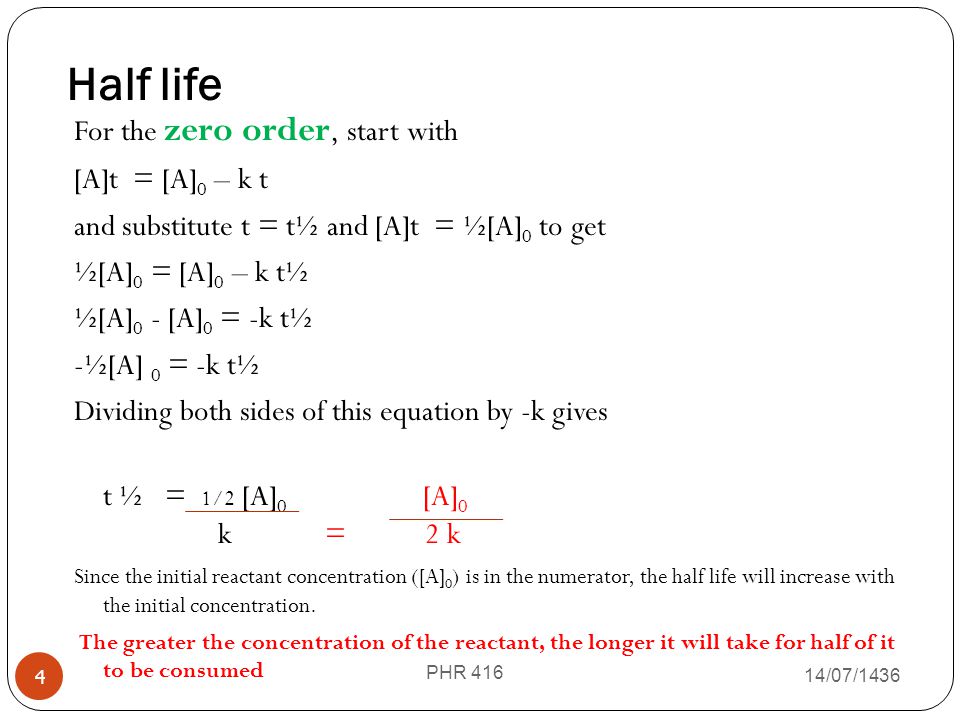
Half life formula for zero order reaction The half-life of a reaction t_12 is the amount of time needed for a reactant concentration to decrease by half compared to its initial. For a general reaction. Which is the required equation for the half-life of zero order reactions.
It is clearly visible from the above equation that the half-life of the reaction is dependent on the rate constant as well as the initial concentration of the reactant. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k. For a zero-order reaction the half-life equation is given as.
The equation given above shows that the half-life is dependent on the rate constant and the. The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. In a reversible reaction the energy of.
The rate constant k for the reaction or enough information to determine it. The half-life is the time required for a quantity to fall to half its initial value as measured at the beginning of the time period. How do you find the order of a half-life reaction.
The half-life formula for various reactions is given below. If we know the integrated rate laws we can determine the half. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant.
For a first zero order. It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction. As for all reaction orders the half-life for a zero.
To use this online calculator for Half Life of Second Order Reaction enter Reactant Concentration CA Rate Constant for Second Order Reaction Ksecond and hit the calculate button. The half-life of a first-order reaction is given as t 12 0693k. As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law.
However for catalytic reactions at low concentrations they are no longer linear since they stop being zero-order reactions. The half-life of a species is the time it takes for it to. The half-life formula for a reaction depends upon the order of a reaction.
The half-life of a second-order. For a second-order reaction t12 t 1 2 is inversely proportional to the concentration of the reactant and the half-life increases as the reaction proceeds because the. The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction.
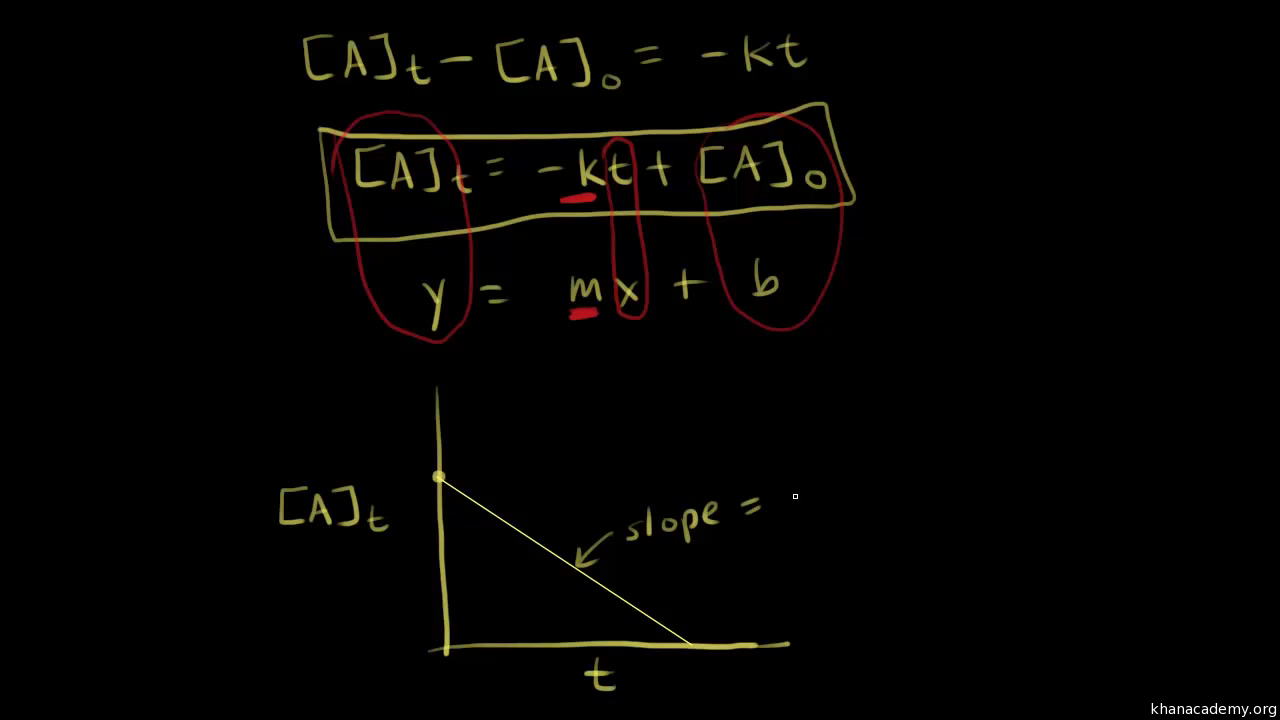
A A 0 - kt. The order of the reaction or enough information to determine it. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
Now replacing t with half-life t12 in. The half-life equation for a zero-order reaction is t12A02k t 1 2 A 0 2 k. A_t ktA_0.
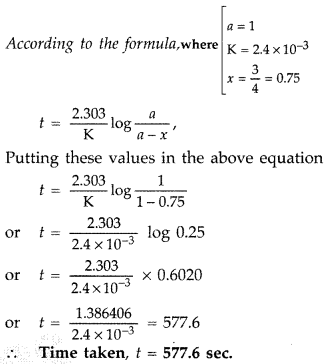
Thus the half-life of a zero order reaction can be determined by taking the final concentration of the reacting species as half of its initial concentration and applying this. From the above-integrated equation we have. Then injection of maximum activity of radioactive substance that can be injected will be Answer.

Derive The Relationship Between Half Life Period And Rate Constant Of A Zero Order Reaction
Zero Order Reaction With Calculus Video Khan Academy

Rate Equation And Order Of Reaction
Important Questions For Class 12 Chemistry Chapter 4 Chemical Kinetics Class 12 Important Questions Learn Cbse
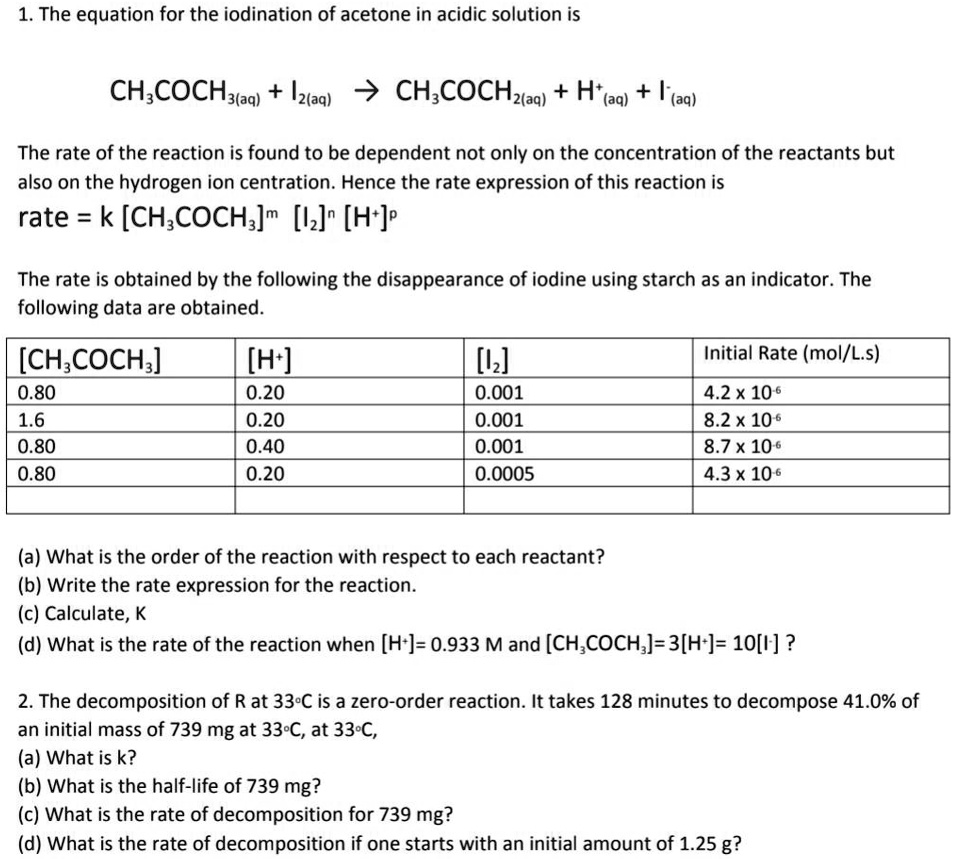
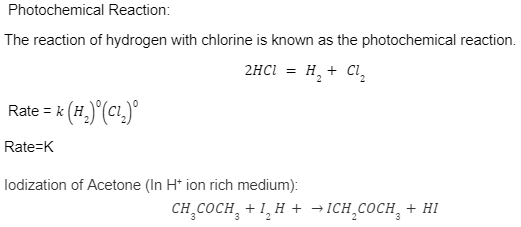
Solved 1 The Equation For The Iodination Of Acetone In Acidic Solution Is Ch Cochyaq Iztaa Ch Cochzaq H Aq Aq The Rate Of The Reaction Is Found To Be Dependent Not Only
Units Of Rate Constant K Chemistry Steps
Using Graphs To Determine Rate Laws Rate Constants And Reaction Orders

Half Life Expressions Chemistnate
M13q6 Integrated Rate Laws And The Method Of Half Lives Chem 103 104 Resource Book
Half Life Introduction To Chemistry Course Hero

Solved Half Life Equation For First Order Reactions T 2 Chegg Com

5 Ways To Calculate Half Life Wikihow